Presented by: Tom L. Rosamond, MD, MSc, FAHA, FACC, FASE
Dr. Thomas Rosamond is Board Certified in Cardiovascular Disease. Dr. Rosamond is the Division Chief of Cardiovascular Imaging and Medical Director of Non-Invasive Imaging Services at The University of Kansas Health System. He earned his Postdoctoral Fellowship in Cardiovascular Magnetic Resonance Imaging at Washington University School of Medicine -Barnes Jewish Hospital in St. Louis, Missouri.
Silent Threat: Decoding the Complexity of Cardiac Transplant Vascular Disease
Cardiac allograft vasculopathy (CAV) is a formidable and often silent threat to patients who have undergone heart transplantation. As one of the most significant causes of late graft failure, CAV remains a primary contributor to the need for re-transplantation and contributes to long-term mortality in transplant recipients. However, diagnosing this condition early and accurately presents significant challenges. The complexity of CAV demands an approach that goes far beyond traditional diagnostic paradigms to ensure timely interventions that can improve patient outcomes. This article delves into the diagnostic complexities of CAV, highlighting the need for sophisticated, multi-layered strategies that enhance early detection and provide precise prognostic insights.
The Diagnostic Imperative: Looking Beyond Surface Findings
One of the key challenges in managing CAV is the fact that the disease often presents with minimal, non-specific symptoms, especially in its early stages. Traditional diagnostic methods, such as catheterization and angiography, may fail to detect critical changes in vascular health, especially when microvascular disease is involved. This limitation is particularly concerning since early and accurate detection of CAV is crucial for managing the condition effectively and improving long-term outcomes for transplant recipients.
For many patients, by the time the disease becomes clinically evident through conventional diagnostics, it may be too late for interventions to have a meaningful impact. Therefore, healthcare providers must adopt a more nuanced diagnostic approach that looks beyond the surface findings and includes advanced imaging and flow assessment strategies to detect the earliest signs of disease.
Key Diagnostic Challenges
The Limitations of Conventional Diagnostics
Traditional diagnostic methods, while essential in the overall assessment of cardiac allografts, often fall short in detecting the full scope of CAV.
- Catheterization Reports May Not Reveal the Full Clinical Picture: Conventional coronary angiography, commonly used in catheterization procedures, typically identifies large vessel disease but may fail to detect subtle microvascular damage, which plays a pivotal role in the progression of CAV.
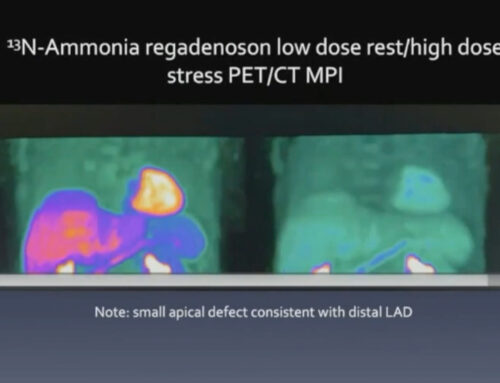
- Microvascular Disease Often Goes Undetected: The small blood vessels of the heart are often the most affected by CAV, yet these changes are difficult to visualize using traditional methods. Microvascular involvement can lead to a severe reduction in myocardial perfusion, even without detectable epicardial disease.
- Critical Prognostic Indicators Can Be Overlooked: The standard diagnostic approach often focuses on epicardial coronary vessels, overlooking other key markers of disease progression such as myocardial flow reserve, which is a critical predictor of patient outcomes.
Diagnostic Precision: A Critical Paradigm Shift
The evolving landscape of cardiac transplant care is beginning to recognize the importance of a more comprehensive diagnostic approach. A shift towards advanced imaging techniques and sophisticated flow reserve quantification is allowing for greater precision in diagnosing CAV, particularly in its early stages.
Advanced Assessment Strategies
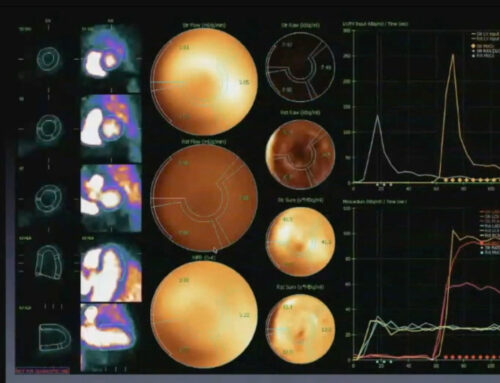
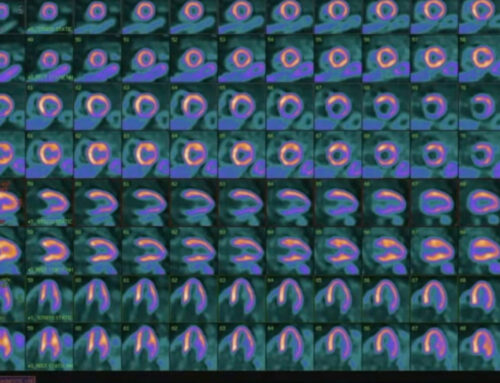
- Comprehensive Imaging Beyond Standard Catheterization: In contrast to traditional angiography, advanced imaging modalities such as intravascular ultrasound (IVUS), magnetic resonance imaging (MRI), and positron emission tomography (PET) offer greater insights into the structure and function of both large and small vessels. These techniques allow clinicians to assess coronary microvascular function, identify early signs of CAV, and visualize non-obstructive coronary changes that would otherwise go unnoticed.
- Detailed Flow Reserve Quantification: Flow reserve—measured through techniques like Doppler flow measurements or positron emission tomography—is an essential tool for assessing the functional capacity of the heart’s blood vessels. This technique evaluates how well blood is flowing through the coronary arteries under stress, revealing subtle functional impairments that are not visible through standard angiography.
- Identification of Global Microvascular Involvement: Microvascular dysfunction in CAV is a global phenomenon that affects the entire coronary bed, not just specific vessels. Identifying this global involvement is crucial, as it can provide valuable insights into the progression of disease and help guide the treatment approach.
Prognostic Insights
Flow Reserve: A Critical Predictor
One of the most powerful prognostic tools for patients with CAV is myocardial flow reserve (MFR), a measure of coronary vasodilatory capacity. MFR is a sensitive indicator of the heart’s ability to increase blood flow during periods of stress, and its reduction is an early sign of worsening graft health.
- Myocardial Flow Reserve < 2 Indicates:
- Accelerated Risk of Death: When flow reserve falls below 2, patients face an increased risk of mortality. This threshold is an early indicator that the heart is not receiving adequate blood flow to meet the demands of the body, often signaling the need for more aggressive management or re-transplantation.
- Increased Re-Transplantation Likelihood: A reduced flow reserve indicates advanced disease, and patients with this finding are more likely to require re-transplantation within a shorter period.
- Rapid Disease Progression: A decrease in flow reserve often correlates with rapid progression of CAV, which can lead to graft failure if not addressed promptly.
Long-Term Risk Stratification
For patients with CAV, long-term risk stratification is essential for guiding management decisions. A flow reserve of < 1.5 is associated with an alarmingly high risk of death or re-transplantation, with 60% of patients facing this outcome within five years. This makes MFR a crucial tool for clinicians in identifying high-risk patients and initiating timely interventions.
Importantly, flow reserve < 1.5 is an independent risk factor for adverse outcomes, meaning that even in the absence of significant epicardial coronary disease, a reduced flow reserve is predictive of poor long-term survival and function.
Clinical Recommendations
Diagnostic Best Practices
To effectively manage CAV, healthcare providers should adopt the following best practices:
- Always Verify: Clinicians should review original catheterization films rather than relying solely on written interpretations. Subtle vascular changes may be missed in routine reports, and reviewing the images themselves may reveal early signs of CAV.
- Comprehensive Screening: Implement advanced flow reserve assessments as part of routine screening for heart transplant recipients. These assessments provide critical insights into graft function that are not captured by standard angiography.
- Consider Microvascular Evaluation in All Transplant Patients: Since microvascular dysfunction is a hallmark of CAV, all transplant patients should undergo an evaluation for microvascular disease, even if they do not exhibit overt epicardial coronary disease. Early identification of microvascular involvement can help guide more targeted therapeutic strategies.
- Recognize the Global Nature of CAV: CAV affects not just the epicardial arteries but also the microvascular network. Comprehensive screening should include evaluations for both macrovascular and microvascular changes to ensure a complete understanding of the patient’s graft health.
Emerging Perspectives
The future of cardiac transplant care lies in precision diagnostic technologies that enable earlier detection of microvascular changes and more personalized risk stratification. As imaging technologies continue to evolve, we can expect increasingly accurate and non-invasive methods to diagnose CAV and monitor disease progression. Personalized treatment plans, tailored to the specific characteristics of each patient’s disease, will lead to better long-term outcomes and improved graft survival.
Cardiac allograft vasculopathy remains one of the most challenging complications in heart transplant care, but a sophisticated, technology-driven approach can improve diagnosis and management. By embracing advanced imaging, detailed flow reserve quantification, and a comprehensive view of microvascular health, clinicians can detect CAV earlier and offer more effective treatments. Moving beyond traditional diagnostic paradigms is crucial for improving outcomes, enhancing patient care, and ultimately prolonging the life of heart transplant recipients.